



Next: Electronic Structure of
Up: Introduction
Previous: Introduction
An ideal heterojunction consists of a semiconductor crystal (in the
sense of a regular network of chemically bonded atoms) in which there
exists a plane across which the identity of the atoms participating
in the crystal changes abruptly.
In practice, the ideal structure is approached quite closely in some
systems. In high-quality Al Ga
Ga As-GaAs heterojunctions it
has been found that the interface is essentially atomically abrupt [6].
There is an entire spectrum
of departures from the ideal structure, in the form of crystalline
defects. The most obvious cause of such defects is mismatch between
the lattices of the participating semiconductors. The lattice
constants of GaAs and AlAs are nearly equal, so these materials fit
together quite well. In contrast, the lattice constants of Si and Ge
differ significnatly, so that over a large area of the heterojunction
plane, not every Si atom will find a Ge atom to which to bond. This
situation produces defects in the form of dislocations in one or the
other of the participating semiconductors, and such dislocations
usually affect the electrical characteristics of the system by creating
localized states which trap charge carriers. If the density of such
interfacial traps is sufficiently large, they will dominate the
electrical properties of the interface. This is what usually happens
at poorly controlled interfaces such as the grain boundaries in
polycrystalline materials. The term heterojunction is usually
reserved for those interfaces in which traps play a negligible role.
As-GaAs heterojunctions it
has been found that the interface is essentially atomically abrupt [6].
There is an entire spectrum
of departures from the ideal structure, in the form of crystalline
defects. The most obvious cause of such defects is mismatch between
the lattices of the participating semiconductors. The lattice
constants of GaAs and AlAs are nearly equal, so these materials fit
together quite well. In contrast, the lattice constants of Si and Ge
differ significnatly, so that over a large area of the heterojunction
plane, not every Si atom will find a Ge atom to which to bond. This
situation produces defects in the form of dislocations in one or the
other of the participating semiconductors, and such dislocations
usually affect the electrical characteristics of the system by creating
localized states which trap charge carriers. If the density of such
interfacial traps is sufficiently large, they will dominate the
electrical properties of the interface. This is what usually happens
at poorly controlled interfaces such as the grain boundaries in
polycrystalline materials. The term heterojunction is usually
reserved for those interfaces in which traps play a negligible role.
From the above considerations one would logically conclude that closely
matching the lattice constants of the participating semiconductors
(good ``lattice matching'') is a necessary condition for the
fabrication of high-quality heterojunctions. Indeed this was the
generally held view for many years, but more recently high-quality
heterojunctions have been demonstrated in ``strained-layer'' or
pseudomorphic systems [7,8].
The essential idea is that if one of the
semiconductors forming a heterojunction is made into a sufficiently thin
layer, the lattice mismatch is accommodated by a deformation (strain) in
the thin layer. With this approach it has proved possible to make
high-quality heterojunctions between Si and Ge Si
Si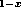 alloys [4].
alloys [4].
Heterostructures are generally fabricated by an epitaxial growth process.
Most of the established epitaxial techniques have been applied to the growth
of heterostructures. These include Molecular Beam Epitaxy (MBE) [6] and
Metalorganic Chemical Vapor Deposition (MOCVD) [9]. Liquid Phase Epitaxy (LPE) is
an older heterostructure technology, which has largely been supplanted by
by MBE and MOCVD because it does not permit as precise control of the
fabricated structure.
The examples of heterojunctions cited so far involve chemically similar
materials, in the sense that both constituents contain elements from the
same columns of the periodic table. It is possible to grow heterojunctions
between chemically dissimilar semiconductors (those whose constituents come
from different columns of the periodic table), such as Ge-GaAs and GaAs-ZnSe,
and such junctions were widely studied early in the development of
heterostructure technology [10]. There are, however, a
number of problems with such junctions. Based upon simple models of the
electronic structure of such junctions, one would expect a high density of
localized interface states due to the under- or over-satisfied chemical bonds
across such a junction [11,12] . More significantly, perhaps, the constituents
of each semiconductor act as dopants when incorporated into the
other material.
Thus any interdiffusion across the junction produces electrical effects
which are difficult to control. For these reasons, most recent work has
focused upon chemically matched systems.
If a heterojunction is made between two materials for which there exists a
continuum of solid solutions, such as between GaAs and AlAs
(as Al Ga
Ga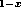 As exists for all x such that
As exists for all x such that 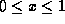 ), the
chemical transition need not occur abruptly. Instead, the heterojunction
may be ``graded'' over some specified distance. That is, the composition
parameter x might be some continuous function of the position. Such
heterojunctions have desirable properties for some applications.
), the
chemical transition need not occur abruptly. Instead, the heterojunction
may be ``graded'' over some specified distance. That is, the composition
parameter x might be some continuous function of the position. Such
heterojunctions have desirable properties for some applications.




Next: Electronic Structure of
Up: Introduction
Previous: Introduction
William R. Frensley
Sun May 21 16:29:20 CDT 1995
 Ga
Ga As-GaAs heterojunctions it
has been found that the interface is essentially atomically abrupt [6].
There is an entire spectrum
of departures from the ideal structure, in the form of crystalline
defects. The most obvious cause of such defects is mismatch between
the lattices of the participating semiconductors. The lattice
constants of GaAs and AlAs are nearly equal, so these materials fit
together quite well. In contrast, the lattice constants of Si and Ge
differ significnatly, so that over a large area of the heterojunction
plane, not every Si atom will find a Ge atom to which to bond. This
situation produces defects in the form of dislocations in one or the
other of the participating semiconductors, and such dislocations
usually affect the electrical characteristics of the system by creating
localized states which trap charge carriers. If the density of such
interfacial traps is sufficiently large, they will dominate the
electrical properties of the interface. This is what usually happens
at poorly controlled interfaces such as the grain boundaries in
polycrystalline materials. The term heterojunction is usually
reserved for those interfaces in which traps play a negligible role.
As-GaAs heterojunctions it
has been found that the interface is essentially atomically abrupt [6].
There is an entire spectrum
of departures from the ideal structure, in the form of crystalline
defects. The most obvious cause of such defects is mismatch between
the lattices of the participating semiconductors. The lattice
constants of GaAs and AlAs are nearly equal, so these materials fit
together quite well. In contrast, the lattice constants of Si and Ge
differ significnatly, so that over a large area of the heterojunction
plane, not every Si atom will find a Ge atom to which to bond. This
situation produces defects in the form of dislocations in one or the
other of the participating semiconductors, and such dislocations
usually affect the electrical characteristics of the system by creating
localized states which trap charge carriers. If the density of such
interfacial traps is sufficiently large, they will dominate the
electrical properties of the interface. This is what usually happens
at poorly controlled interfaces such as the grain boundaries in
polycrystalline materials. The term heterojunction is usually
reserved for those interfaces in which traps play a negligible role.
 Si
Si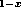 alloys [
alloys [ Ga
Ga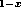 As exists for all x such that
As exists for all x such that 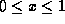 ), the
chemical transition need not occur abruptly. Instead, the heterojunction
may be ``graded'' over some specified distance. That is, the composition
parameter x might be some continuous function of the position. Such
heterojunctions have desirable properties for some applications.
), the
chemical transition need not occur abruptly. Instead, the heterojunction
may be ``graded'' over some specified distance. That is, the composition
parameter x might be some continuous function of the position. Such
heterojunctions have desirable properties for some applications.