Electro-
magnetic
Radiation
James ClerkMaxwell

MS Encarta '97
The other things you associate with radiation, from the glow of embers in a fire to light from the most distant galaxies, is electromagnetic as was explained by James Clerk Maxwell over a century ago. His famous four equations of electricity and magnetism implied the mutual propagation of oscillating electric and magnetic fields (changing electric fields make magnetism - witness an electromagnet - while changing magnetic fields make electricity - witness electric generators). As long as these fields were perpendicular to one another, they merrily recreated one another off to infinity...or until they were absorbed by matter.
Those mated fields were light, and their sinusoidal oscillations explained all of last century's interesting light properties, such as diffraction (rainbows) and reflection (mirrors).
Yes, light was definitely a wave.
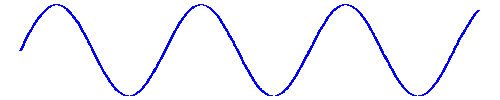
And the spacing between peaks was the "wavelength" symbolized by l. And as it travelled past you, the time between peaks was the "period" symbolized by t. And since the velocity of anything is the distance travelled, l, in a given time, t, the velocity of the wave, that is, the speed of light, is obviously v = l/t.
The Speed
of Light
Albert Einstein
MS Encarta '97
c is special as a velocity since Einstein positted that nothing can exceed the speed of light. You might think that at c = 3×108 m s-1 nothing would need to exceed the speed of light, but NASA would like to. It would permit exploration of other solar systems in a person's lifetime, but even at the speed of light, the nearest star, a Centuri, is a 9 year round trip away. That's a lot of Twinkies and Coke!
Relativity aside, we have a simple way of converting between the wavelength and the frequency of light, viz., l = c/n and vice versa. That's important because spectroscopic measurements of light are made in wavelength, but energy of light is proportional to its frequency!
Planck's
Equation
Max Planck
MS Encarta '97
This relation and the prism (or diffraction grating) which casts white light into its components with known wavelengths bent by known angles, give us the tools we need to peer into the inner workings of atoms! Not merely check their size (atomic microscopy) but rather determine experimentally their internal energy states! How? By giving them a kick and seeing what light falls out.
And the kicker is that the light that falls out is not smeared out over many wavelengths but rather concentrated into several lines in atomic spectra. Those lines mean incredibly precise energy differences between atomic states since any energy the atom loses, the photon gains (by conservation of energy).
Hydrogen
Spectrum
The visible lines in the
spectrum of hydrogen.
(The violet line is
very weak.)
 Niels Bohr
Niels Bohr
And Bohr's development also worked for He+, Li2+, Be3+, etc. ... all the ions with only one electron on them! But nothing else. Bummer.
Multi-
electron
Atoms
They were instead smeared out into waves!
This was first guessed by deBroglie who prediced that a "matter wave" would have a wavelength inversely proportional to its momentum, l = h/mv, where (lo and behold) the constant of (inverse) proportionality turned out to be Planck's!!! (Of course it's not a coincidence.)
Matter
Waves
Nobel Laureate Richard Feynman said it best when he suggested "Do not keep saying to yourself, if you can possibly avoid it, 'But how can it be like that?' because you will get 'down the drain' into a blind alley from which nobody has escaped. Nobody knows how it can be like that."
In essence, all matter gets where its wave can go but appears as a particle when it gets there. Until it gets there, it is wherever its (diffuse) matter wave is. Worse still, it makes its own matter wave, like God surfing.
So even though that's screwy...because nothing in our day-to-day world acts that way, it is nonetheless true of atomic sized things. These matter wave phenomena don't trouble us non-atomic critters because our masses are so large that our deBroglie wavelengths vanish well below the dimensions of a nucleus let alone an atom! Such small wavelengths don't diffract on anything, so none of us ever spontaneously breaks up into a rainbow. Pity; sounds like fun.
Electron
Orbitals
Electron waves, like light waves, have nodes, but while light is in constant motion (c), electrons trapped in atoms are stationary waves with fixed nodal patterns.
Principal
Quantum
Number
But where are these nodes? To begin with, they are radial surfaces within the electron matter wave (centered on the nucleus) where the wave has zero amplitude. If n=3, there are n-1=2 such spherical nodes, but for n=1, there's none at all, and that matter wave just dies out as one walks away from the nucleus.
Angular
Quantum
Number
Magnetic
Quantum
Number
ml is actually the (quantized) shadow (projection) of the electron's angular momentum, l, along that imaginary external magnetic field. In a vector sense, ml is l's component along that direction. Since the component can never be larger than its vector, ml never exceeds l and never projects more negative than - l. Thus, ml takes on the 2l+1 integer values from - l to + l.
Spin
Quantum
Number
(Why they spin has to do with Einstein's prohibition that nothing travels faster than light. If l=0, the electron has no angular momentum and should be able to fall into the nucleus. But it's infinitely attracted there as its distance to the nucleus goes to zero. Infinitely negative potential energy has to be balanced by infinitely positive kinetic energy, requiring infinite speeds! Sorry, says Einstein, c is the speed limit; you'll have to go around the nucleus not through it. ms is the angular momentum that the electron carries with it to avoid having to go through!)
Pauli
Principle
However, it is probably the single most important reason why we exist at all! We are creatures of Chemistry. Chemistry is a creature of the wonderful differences between atoms. If the Pauli Principle were false, all atoms would be the same chemically and they'd never bond to make molecules! No molecules? No Life!