Lecture Notes from CHM 1341
14 June 1996
The Periodic Table
Now we're entering Chemical Taxonomy. You remember Biology's taxonomy, i.e., classification scheme: Kingdom, Phylum, etc.? Well, Chemistry has its taxonomy of the elements: Groups and Periods. Biology's taxonomy is now known to be the consequence of Biology's primary particles: genes. Likewise, Chemistry's taxonomy will be found to arise from its primary particles: electrons. (Physics has its own, and it arises from even smaller "primary" particles called "quarks.")
It was Mendele'ev who literally charted the course of the elements, building his table with rows where heavier elements succeeded lighter ones until the completion of the row was signalled by finding an element with the same properties as the one at the beginning. That new one began the next row so as to lie directly beneath its chemical soulmate.
The rows are called Periods and the columns Groups. So all the elements in a row fall one after the other in order of their increasing atomic mass...well at least they did for Mendele'ev; modern tables have them succeed one another by atomic number (Z) instead; that order makes more sense, given that electrons will control the destiny of chemicals and nuclear charge controls the electrons! The elements in the same column (Group) are grouped together by the similarity of their chemistry--all the corrosive halogens in Group VII (pastelle orange) and all the highly combustible alkali metals in Group I (bright orange), for example.
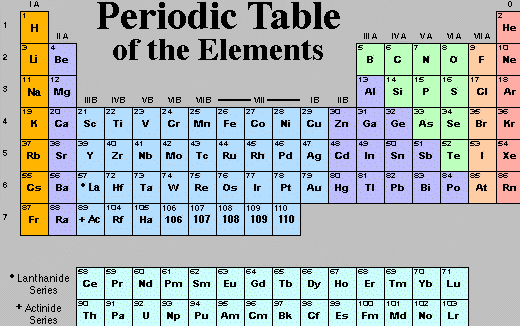
Clicking on the table above takes you to the Los Alamos National Laboratories (got your clearance?), the source of the image, where you can click on its elements for more information on each. Don't forget to click the BACK button to return here!
The power of the Periodic Table was immediately evident in that many of its elements were missing in Mendele'ev's time. So he had the privilege of predicting the discovery of them...and being right. Not only did he know what masses the missing elements should have and what chemistry they'd share with known elements but he could also take advantage of many other atomic properties which vary regularly, indeed periodically, across and down the table. Boiling and melting points are among these. So Mendele'ev could point researchers in the direction of an element with the physical and chemical properties of "eka-silicon," the element which should lie below Si; it was found and named Germanium by its German discoverer, Winkler (1886). (Germany was THE place to be doing chemistry then; it was where the dye industry became high tech.)
The regularity of the table is also evident from the numerology of its period lengths. The rationale for this numerology is a subject for Chapter 6, but its symmetry can be appreciated here. Period 1 consists of 2 elements. Periods 2 and 3 of 2+6=8 where that 6 is 4 more than 2. Then Periods 4 and 5 have 2+6+10=18 where 10 is 4 more than 6. So we'd suspect Periods 6 and 7 should have 2+6+10+14=32, and they do if you notice that a piece of each period is printed below the table labelled "Lanthanide" and "Actinide" series, respectively. If we didn't run out of elements (their nuclei become too unstable to survive), the next Period would have 2+6+10+14+18=50 elements in it.
In fact, those period lengths, 2, 8, 18, 32, 50, etc., are just 2 n² where n is the shell of electrons being filled.
More interesting than mystic numerology are the gross features of the table. All the metals are collected on the left...as various blues in the table above except for the hot orange of the alkali metals. The nonmetals are gathered at the right. The diagonal stair step separating the green from the blue above is where metallic properties (luster, ductility, malleability, conductivity, etc.) segue into the nonmetals (electrically insulating, lusterless, friable, etc.). It is on either side of that dividing line that the semimetals, sometimes called "metalloids," lie. They exhibit properties between those of metals and nonmetals. Metalloids are the "semiconductors" (now they do and now they don't) of microelectronics fame.
Gross features of chemical properties can be found in the table as well. Elements on the left form alkaline oxides (thus "alkali metals" and "alkaline earth" elements of Groups I & II) while elements on the right form acidic oxides. Predictably, those along the metalloid dividing line can't make up their minds about that either; they form "amphoteric" oxides, only weakly acidic or basic.
But perhaps the most astounding regularity in the Periodic Table is the regularity of valence. Ignoring for the moment the transition metals (called IB through VIIIB above) and fixating instead on the Main Groups (IA through VIIIA in that table's parlance), we find that the valence of the elements is the same as the Group number up through IV and then becomes 8 minus the Group number thereafter. This generates the sequence 1, 2, 3, 4, 3, 2, 1, 0, where 0 (the "salmon-colored" noble or rare gases) means "no valence," that is "unreactive"...they do not combine with other elements or even themselves. Aloof. Perhaps that's how you stay rare and noble?
Knowing this, oxygen's valence is predicted to be 8-VI=2, assuming you can do mixed Arabic and Latin arithmetic! (Don't say Chemistry never taught you a skill.) Armed with oxygen's valence of 2, we predict the following oxides from Period 2:
Li2O BeO B2O3 CO2 N2O3 O2 F2O
Of course, we already know that there are more; CO and NO (and many more) exist, but Mendele'ev could use this periodicity to predict the compounds that searchers for new elements should seek!
Return to the CHM 1341 Lecture Notes or Go To Next or Previous Lectures.
Chris Parr
University of Texas at Dallas
Programs in Chemistry, Room BE3.506
P.O. Box 830688 M/S BE2.6 (for snailmail)
Richardson, TX 75083-0688
Voice: (214) 883-2485
Fax: (214) 883-2925
BBS: (214) 883-2168 (HST) or -2932 (V.32bis)
Internet: parr@utdallas.edu (sends Chris e-mail.)
Last modified 2 September 1996.
