Lecture Notes from CHM 1341
1 July 1996
Quantum Chemistry
So light behaves as both a wave (continuously varying oscillation which "feels" the environment over all its wavelength) and a particle (a entity at some point in space sensing only objects and fields there). In case the ramping stamping incongruity of that hasn't hit you yet, these are incompatible traits. It means that we haven't an ordinary world model ("Gee...it's just like...) to cling to. So we can give it a new name ("wavicle?") and accept it for what it is and does. If we persist in living in this dualistic paradox, we can hedge our bets by saying, depending on the experiment, light will act either as a wave or as a particle.
 If the experiment is a diffraction one (as with the one at the right), light obliges by acting as a wave. If it's a particle-type experiment (knocking an electron off potassium, for example), light behaves like a particle. All these would be a little easier to swallow if matter didn't do the same thing!
If the experiment is a diffraction one (as with the one at the right), light obliges by acting as a wave. If it's a particle-type experiment (knocking an electron off potassium, for example), light behaves like a particle. All these would be a little easier to swallow if matter didn't do the same thing!
But it does!
 While Bohr was sure he'd got his hydrogenic electron orbits right because from them he could calculate the experimental spectra dead on, de Broglie and others knew that the notion of little electron corpusles whizzing around like planets was entirely inconsistent with the Davisson-Germer experiment in which electron beams DIFFRACTED off crystals just like light! So while that pattern on the left actually came from a laser beam in a "double slit" experiment, it could just as well have been an electron beam...as long as all the electrons had the same energy. Because, if you changed the energy, the pattern changed as if you'd altered a wavelength! As far as de Broglie was concerned, that's exactly what you'd done; in no time at all he had concluded that there were "wavelengths" associated with matter via its momentum, p. (That's little "p" since big "P" is pressure.)
While Bohr was sure he'd got his hydrogenic electron orbits right because from them he could calculate the experimental spectra dead on, de Broglie and others knew that the notion of little electron corpusles whizzing around like planets was entirely inconsistent with the Davisson-Germer experiment in which electron beams DIFFRACTED off crystals just like light! So while that pattern on the left actually came from a laser beam in a "double slit" experiment, it could just as well have been an electron beam...as long as all the electrons had the same energy. Because, if you changed the energy, the pattern changed as if you'd altered a wavelength! As far as de Broglie was concerned, that's exactly what you'd done; in no time at all he had concluded that there were "wavelengths" associated with matter via its momentum, p. (That's little "p" since big "P" is pressure.)
Momentum is what was transferred to the walls of a vessel when gas molecules bounced off it, remember? It has an expression even simpler than energy; that is, p=mv with units kg m/s. Play with the algebra a little and p²=2mE (where E here is kinetic energy, ½mv²).
de Broglie concluded that a matter wave had to have a wavelength of W=h/p, where h is Planck's constant again.
As soon as this was known, the question of unique energy levels in atoms started to make sense. If electrons behaved (until you captured one) as waves, and they were spread out all over the atom, the only sorts of wavelengths which were tolerable were ones which didn't cancel themselves out! Ones with wavelengths which put all parts of the wave in sync with itself (crests on crests, for example) were constructive rather than destructive (crest on trough) and could be sustained as "standing waves," as the physicists call them. And when Schödinger put the numbers to such standing waves, out popped the experimental energy levels once again...and a new kind of mechanics was born. Since it dealt with discrete quantities, like energy levels, it became known as quantum mechanics, and it rules any system in which de Broglie's matter wavelengths are comparable to the constraints imposed by the physical environment. That means molecules and smaller critters.
The orbital (not orbit) shapes which fall out of QM for the H atom are examples of aesthetics in Nature. And thinking about how increasing the energy means decreasing the wavelength, you're led to conclude that each higher energy level must have one more node (place where the wave is zero between crests and troughs) in it. To start off, the ground state has no node; it's "crest everywhere," if you like...spherically symmetric about the nucleus and falling away to nothing (rather quickly, indeed exponentially, but that's another story) with radius from the center. That's the state for shell n=1.
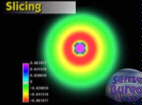 That figure (from U.C. San Diego) at right is one (2s) of the states for n=2. Check out the color scale (coding amplitude) and you'll see that it's got a crest in close and another further out (we're plotting not the wave but it's square, so even troughs are crests) and therefore a nodal surface in between...in the shape of a sphere since this creature's completely spherical. But if you were dividing up space with nodal surfaces, would you confine yourself to only spherical ones? Nature isn't that unimaginative either. Instead of setting the node at some radius, how about setting it at some angle?
That figure (from U.C. San Diego) at right is one (2s) of the states for n=2. Check out the color scale (coding amplitude) and you'll see that it's got a crest in close and another further out (we're plotting not the wave but it's square, so even troughs are crests) and therefore a nodal surface in between...in the shape of a sphere since this creature's completely spherical. But if you were dividing up space with nodal surfaces, would you confine yourself to only spherical ones? Nature isn't that unimaginative either. Instead of setting the node at some radius, how about setting it at some angle?
 That's what's happened to the (2p) orbital on the left. The "angle" is 90 degrees from "up" and it sweeps out a plane perpendicular to the screen, the orbital on both sides of that plane but (since it's nodal) not in it. This plane is perpendicular to the Y axis, say, but turn the picture in your mind 90 degrees about its center and you've constructed yet a third n=2 orbital with a nodal plane perpendicular to the X axis. Rotate it on more time mentally, this time so one of those lobes points at you and the nodal plane is now in the screen (and perpendicular to the Z axis). So there are a total of 4 independent n=2 orbitals; one radial and 3 angular.
That's what's happened to the (2p) orbital on the left. The "angle" is 90 degrees from "up" and it sweeps out a plane perpendicular to the screen, the orbital on both sides of that plane but (since it's nodal) not in it. This plane is perpendicular to the Y axis, say, but turn the picture in your mind 90 degrees about its center and you've constructed yet a third n=2 orbital with a nodal plane perpendicular to the X axis. Rotate it on more time mentally, this time so one of those lobes points at you and the nodal plane is now in the screen (and perpendicular to the Z axis). So there are a total of 4 independent n=2 orbitals; one radial and 3 angular.
It's clear that the representation of this orbital looks solid while the other looks diffuse. Diffuse is a better bet for a 3-dimensional wave, but many textbooks, ours included, use this latter kind of drawing by showing where the wave would take on some particular value by constructing an imaginary surface there.
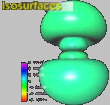 When there's only one node in the wave, it must be either radial or angular, but n=3 has two nodes. Both could be radial or both angular, but the interesting orbital (called 3d) at right has one radial node (separating the inner from outer lobes) and one angular node (90 degrees from Y, slicing the top two from the bottom two lobes). With interesting shapes like this to start with, it's no surprise that atoms can build up into molecules of architectural wonder.
When there's only one node in the wave, it must be either radial or angular, but n=3 has two nodes. Both could be radial or both angular, but the interesting orbital (called 3d) at right has one radial node (separating the inner from outer lobes) and one angular node (90 degrees from Y, slicing the top two from the bottom two lobes). With interesting shapes like this to start with, it's no surprise that atoms can build up into molecules of architectural wonder.
 Just for jollies, the figure at left shows what happens if both nodes for n=3 are angular; chemists call this a 3d orbital.
Just for jollies, the figure at left shows what happens if both nodes for n=3 are angular; chemists call this a 3d orbital.
These have all been examples of the shapes electrons are found in isolated atoms. When atoms approach one another at least their valence electrons are attracted to the (positive) nucleus of their neighbor. So one might expect their orbitals to then reflect this hunger.
Return to the CHM 1341 Lecture Notes or Go To Next or Previous Lectures.
Chris Parr
University of Texas at Dallas
Programs in Chemistry, Room BE3.506
P.O. Box 830688 M/S BE2.6 (for snailmail)
Richardson, TX 75083-0688
Voice: (214) 883-2485
Fax: (214) 883-2925
BBS: (214) 883-2168 (HST) or -2932 (V.32bis)
Internet: parr@utdallas.edu (Click on that address to send Chris e-mail.)
Last modified 30 June 1996.
 If the experiment is a diffraction one (as with the one at the right), light obliges by acting as a wave. If it's a particle-type experiment (knocking an electron off potassium, for example), light behaves like a particle. All these would be a little easier to swallow if matter didn't do the same thing!
If the experiment is a diffraction one (as with the one at the right), light obliges by acting as a wave. If it's a particle-type experiment (knocking an electron off potassium, for example), light behaves like a particle. All these would be a little easier to swallow if matter didn't do the same thing! While Bohr was sure he'd got his hydrogenic electron orbits right because from them he could calculate the experimental spectra dead on, de Broglie and others knew that the notion of little electron corpusles whizzing around like planets was entirely inconsistent with the Davisson-Germer experiment in which electron beams DIFFRACTED off crystals just like light! So while that pattern on the left actually came from a laser beam in a "double slit" experiment, it could just as well have been an electron beam...as long as all the electrons had the same energy. Because, if you changed the energy, the pattern changed as if you'd altered a wavelength! As far as de Broglie was concerned, that's exactly what you'd done; in no time at all he had concluded that there were "wavelengths" associated with matter via its momentum, p. (That's little "p" since big "P" is pressure.)
While Bohr was sure he'd got his hydrogenic electron orbits right because from them he could calculate the experimental spectra dead on, de Broglie and others knew that the notion of little electron corpusles whizzing around like planets was entirely inconsistent with the Davisson-Germer experiment in which electron beams DIFFRACTED off crystals just like light! So while that pattern on the left actually came from a laser beam in a "double slit" experiment, it could just as well have been an electron beam...as long as all the electrons had the same energy. Because, if you changed the energy, the pattern changed as if you'd altered a wavelength! As far as de Broglie was concerned, that's exactly what you'd done; in no time at all he had concluded that there were "wavelengths" associated with matter via its momentum, p. (That's little "p" since big "P" is pressure.) That figure (from U.C. San Diego) at right is one (2s) of the states for n=2. Check out the color scale (coding amplitude) and you'll see that it's got a crest in close and another further out (we're plotting not the wave but it's square, so even troughs are crests) and therefore a nodal surface in between...in the shape of a sphere since this creature's completely spherical. But if you were dividing up space with nodal surfaces, would you confine yourself to only spherical ones? Nature isn't that unimaginative either. Instead of setting the node at some radius, how about setting it at some angle?
That figure (from U.C. San Diego) at right is one (2s) of the states for n=2. Check out the color scale (coding amplitude) and you'll see that it's got a crest in close and another further out (we're plotting not the wave but it's square, so even troughs are crests) and therefore a nodal surface in between...in the shape of a sphere since this creature's completely spherical. But if you were dividing up space with nodal surfaces, would you confine yourself to only spherical ones? Nature isn't that unimaginative either. Instead of setting the node at some radius, how about setting it at some angle? That's what's happened to the (2p) orbital on the left. The "angle" is 90 degrees from "up" and it sweeps out a plane perpendicular to the screen, the orbital on both sides of that plane but (since it's nodal) not in it. This plane is perpendicular to the Y axis, say, but turn the picture in your mind 90 degrees about its center and you've constructed yet a third n=2 orbital with a nodal plane perpendicular to the X axis. Rotate it on more time mentally, this time so one of those lobes points at you and the nodal plane is now in the screen (and perpendicular to the Z axis). So there are a total of 4 independent n=2 orbitals; one radial and 3 angular.
That's what's happened to the (2p) orbital on the left. The "angle" is 90 degrees from "up" and it sweeps out a plane perpendicular to the screen, the orbital on both sides of that plane but (since it's nodal) not in it. This plane is perpendicular to the Y axis, say, but turn the picture in your mind 90 degrees about its center and you've constructed yet a third n=2 orbital with a nodal plane perpendicular to the X axis. Rotate it on more time mentally, this time so one of those lobes points at you and the nodal plane is now in the screen (and perpendicular to the Z axis). So there are a total of 4 independent n=2 orbitals; one radial and 3 angular. When there's only one node in the wave, it must be either radial or angular, but n=3 has two nodes. Both could be radial or both angular, but the interesting orbital (called 3d) at right has one radial node (separating the inner from outer lobes) and one angular node (90 degrees from Y, slicing the top two from the bottom two lobes). With interesting shapes like this to start with, it's no surprise that atoms can build up into molecules of architectural wonder.
When there's only one node in the wave, it must be either radial or angular, but n=3 has two nodes. Both could be radial or both angular, but the interesting orbital (called 3d) at right has one radial node (separating the inner from outer lobes) and one angular node (90 degrees from Y, slicing the top two from the bottom two lobes). With interesting shapes like this to start with, it's no surprise that atoms can build up into molecules of architectural wonder. Just for jollies, the figure at left shows what happens if both nodes for n=3 are angular; chemists call this a 3d orbital.
Just for jollies, the figure at left shows what happens if both nodes for n=3 are angular; chemists call this a 3d orbital.